
On the other hand, even with several proteins and complexes dedicated to folding proteins properly, a fraction of proteins do not achieve native and functional form and are either misfolded or aggregated. Additionally, the cytosolic regions of the transmembrane protein may interact with cytosolic proteins or chaperones to properly fold these domains. If the protein is destined for secretion, it will be released by the chaperones and packaged for travel through the Golgi on to a final destination (such as the plasma membrane or secreted) or move into peroxisomes.
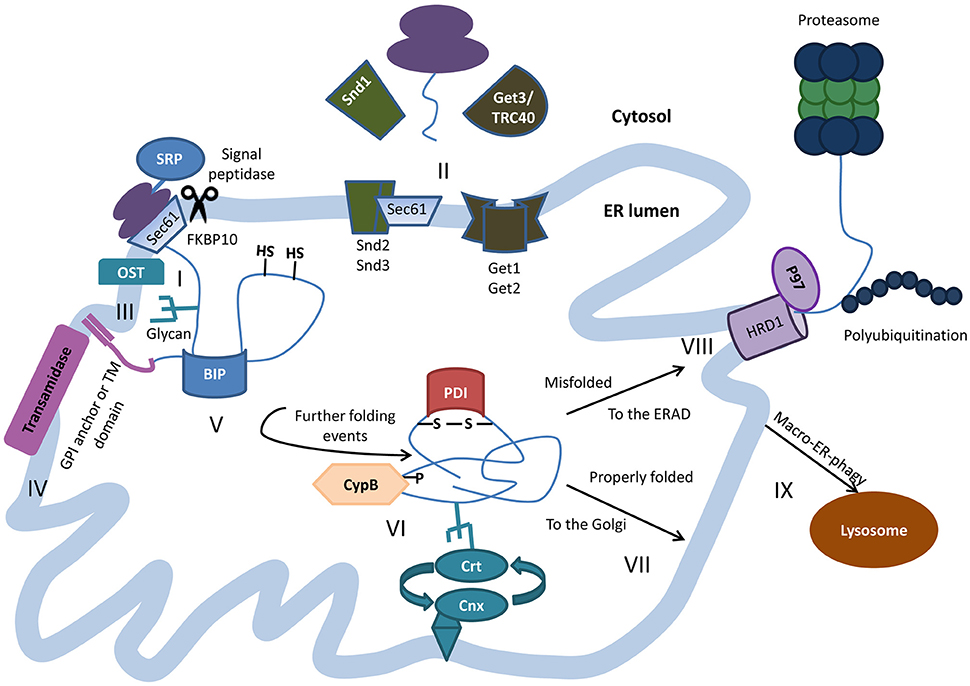

If the protein functions in the ER, for example as a chaperone, then proper folding will commence. At this point the fate of the secretory proteins is determined. These modifications include N-linked glycosylation, disulfide bond formation and oligomerization. įollowing protein synthesis and translocation into the ER lumen, a protein destined for secretion must undergo proper folding and modifications, with the aid of chaperones and folding enzymes. For cytosolic proteins translated on ER-bound ribosomes it is not clear how these mRNAs are recruited to the ER or what populations of ribosomes are utilized to initiate translation, although a recent study indicates that the ER-resident protein p180 may play a role in the translation-independent recruitment of mRNAs to the ER. For mRNAs translated by stably-bound ER ribosomes, mRNAs are released and ribosomes may remain bound to the ER and participate in multiple rounds of translation. Once translation is complete and the signal peptide has been cleaved the ribosomes are released back into the cytosol. If the protein is not destined to be integrated into the membrane, but instead enter the secretory pathway or the lumen of membrane-bound organelles, the protein begins the process of transport. Transmembrane proteins can either contain one hydrophobic stretch of amino acids, and are classified as single pass transmembrane proteins, or contain multiple regions that cross the membrane and are classified as multi-pass transmembrane proteins. At this point the protein will be shifted laterally and become anchored within the phospholipid bilayer where it remains. If the protein is destined to be an integral membrane protein, determined by the presence of a stretch of hydrophobic residues or stop-transfer membrane anchor sequence, translocation will pause.
#Releases new peptides into er lumen srp free#
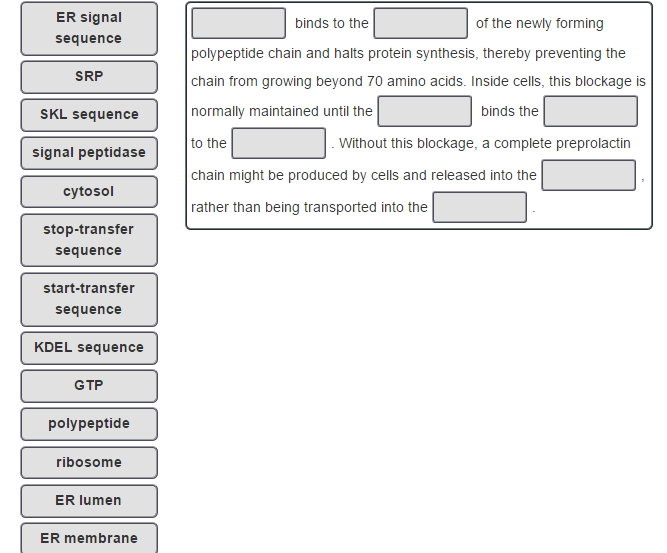
Īlso during this time, or in some cases once translation is complete, a signal peptidase cleaves the short signal peptide allowing the free protein to enter the ER lumen. Translation continues on the ER and the emerging polypeptide can co-translationally enter the ER through the translocon, which is a channel that contains several Sec proteins and spans the lipid bilayer. The complex of mRNA:ribosome:nascent polypeptide:SRP is targeted to the ER where it docks on the SRP receptor. Translation of secretory or integral membrane proteins initiates in the cytosol, then ribosomes containing these mRNAs are recruited to the ER membrane via a signal sequence within the amino terminus of the nascent polypeptide that is recognized and bound by the signal recognition particle (SRP). Protein synthesis requires localization of ribosomes to the cytosolic face of the ER, and the canonical pathway that regulates protein synthesis involves co-translational docking of the mRNA:ribosome complex on the ER membrane. One of the major functions of the ER is to serve as a site for protein synthesis for secreted and integral membrane proteins, as well as a subpopulation of cytosolic proteins.


 0 kommentar(er)
0 kommentar(er)
